Biogeochemical cycles | Carbon Cycle | Nitrogen Cycle | Water Cycle
Biogeochemical Cycles PPT
Water cycle || Nitrogen Cycle || Carbon Cycle
Introduction to Biogeochemical Cycles
Nutrients like water, nitrogen, carbon, phosphorus, etc. cycled through the earth between biotic and abiotic components. This cyclic movement is called Biogeochemical Cycle. Earth sciences, Biogeochemical cycles is a pathway of these (nitrogen, water, carbon, phosphorus) by which these circulate in the different spheres.
The law of mass conservation states that matter cannot be created or destroyed, thus it has to be circulated within the universe. Matter changes in terms of bonding and physical state, but remains within the bounds of this world. The first part of this biogeochemical cycle’s ppt is about the water cycle presentation.
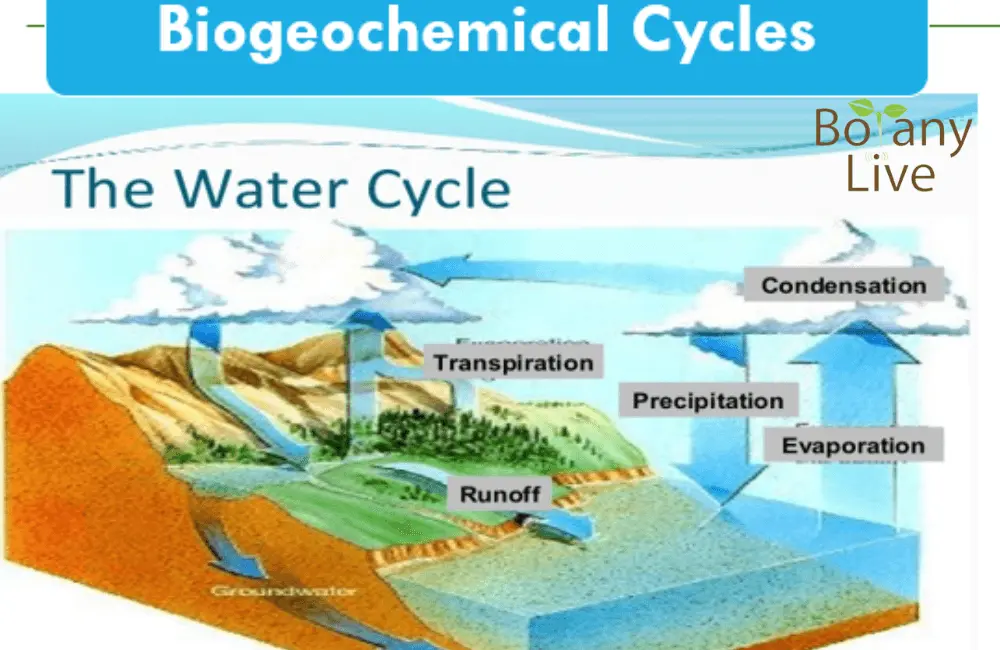
Water Cycle || Hydrological Cycle PPT
(This Water cycle ppt would be helpful even for grades 3 and grade 6)
There are 5 main processes involved in the Water cycle;
1. Evaporation
Water turns into vapours, gaseous form at all temperatures. Water from all the open surface water sources turns into vapours easily. This process depends upon different factors;
- Surface area
- Temperature
- Humidity
- Wind Speed
- Intermolecular Forces in the Liquid
2. Transpiration
Water in the plant and all type of vegetation transpires from the canopy and cover towards the atmosphere. This process helps in the movement of nutrients and minerals from the soil to the aerial parts.
3. Condensation
Water condenses into liquid or gas. This condensation depends upon the ambient temperature. The process of condensation is the root cause of rainfall, snow, hail, or other form of precipitation.
4. Precipitation
This is an important part of the water circulation process. In this part of the water cycle ppt, the slides show how water falls back to earth from the atmosphere. The form of water may be liquid or solid.
5. Run-off
When water falls to earth, it may move to low-lying areas due to the undulated land. This movement of water from the elevated area to the low-lying areas is called run-off. This is gravity-dependent movement. This precious water can be saved from all the losses by vertical farming.
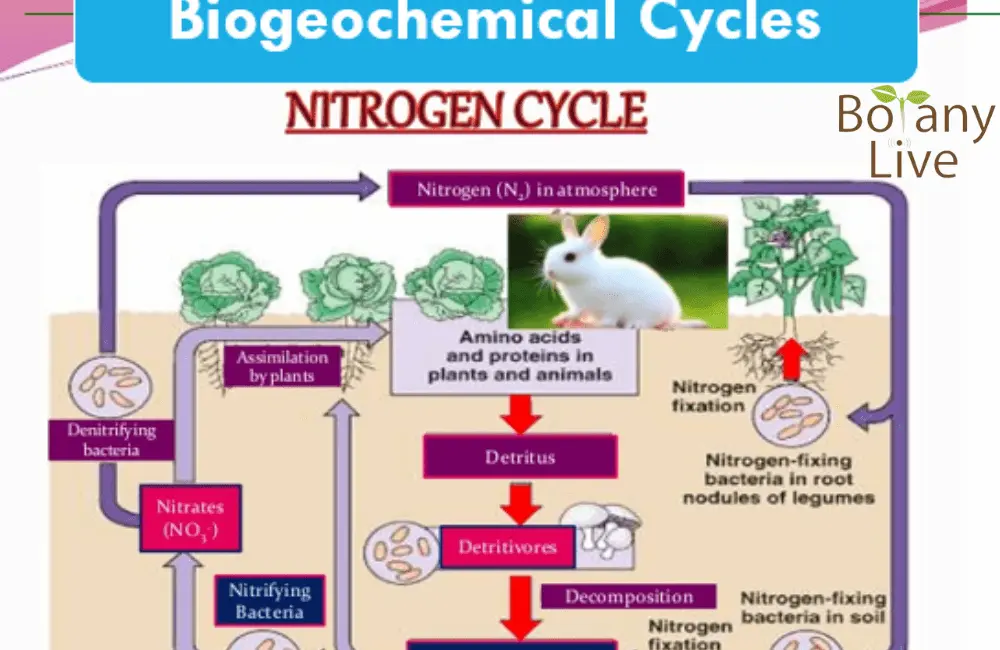
Nitrogen Cycle PPT
Nitrogen gas (N2) is present in the atmosphere at 78% by volume. However, plants are unable to use this molecular form of nitrogen. This atmospheric Nitrogen circulates into the environment in the following forms;
- Nitrates (NO3) – some bacteria and lightening change nitrogen into nitrates
- This nitrate form of nitrogen is taken up by the plants
- Animals eat plants and thus Nitrogen becomes part of their body. Animals excrete nitrogen in the form of excretion and egestion.
- When animals die, this nitrogen is decomposed by decomposers back into the nitrogen gas.
Nitrogen Cycle – Power Point Presentation
What is Nitrogen Fixation?
Symbiotic Nitrogen Fixation – Atmospheric Nitrogen is of no use, it is fixed by free-living bacteria (Azotobacter and Clostridium) and symbiotic (Rhizobium) bacteria. Symbiotic bacteria live in the root nodules. These nodules form plants for association with higher plants. They fix nitrogen for them and in return, get food and protection.
Industrial Nitrogen Fixation – about 30% of nitrogen fixation is due to the industrial Haber-Bosh Process. This process uses high temperatures and pressure and converts nitrogen into ammonia.
What is Nitrification?
Nitrification is an oxidation process by which ammonia is converted into nitrate. This process is carried out by nitrifying bacteria. This is a two-step process. In the first step, Nitrosomonas Bacteria help in the conversion of Ammonia into Nitrite. This nitrite is further converted into Nitrate by Nitrobacter bacteria.
What is Assimilation?
Plants can take nitrogen in the form of nitrite, nitrate, amino acid, or ammonium form. All these forms of nitrogen are ultimately incorporated into amino acids to form protein.
What is Ammonification?
Dead organisms or their waste material such as excreta, and egested material, all are organic forms of nitrogen. Decomposers either fungi or bacteria convert this organic form of nitrogen into the inorganic form of Nitrogen. This conversion process is called Ammonification. As the inorganic form of nitrogen is mineral form, thus, this process is also known as mineralization. This mineralized form is usable by plants only. However, you can use fertigation (field), foliar application (field and labs), or Hydroponic nutrients application for vertical farming.
What is Denitrification?
Denitrification is a reverse process of nitrification. Nitrification is an oxidation process while denitrification is a reduction process. In reduction, the denitrification process takes place in the absence of oxygen. Pseudomonas and Clostridium Bacteria (Anaerobic bacteria) help in this reduction process where the nitrate form of Nitrogen is converted back into molecular nitrogen. Waterlogged condition is helpful in this process.
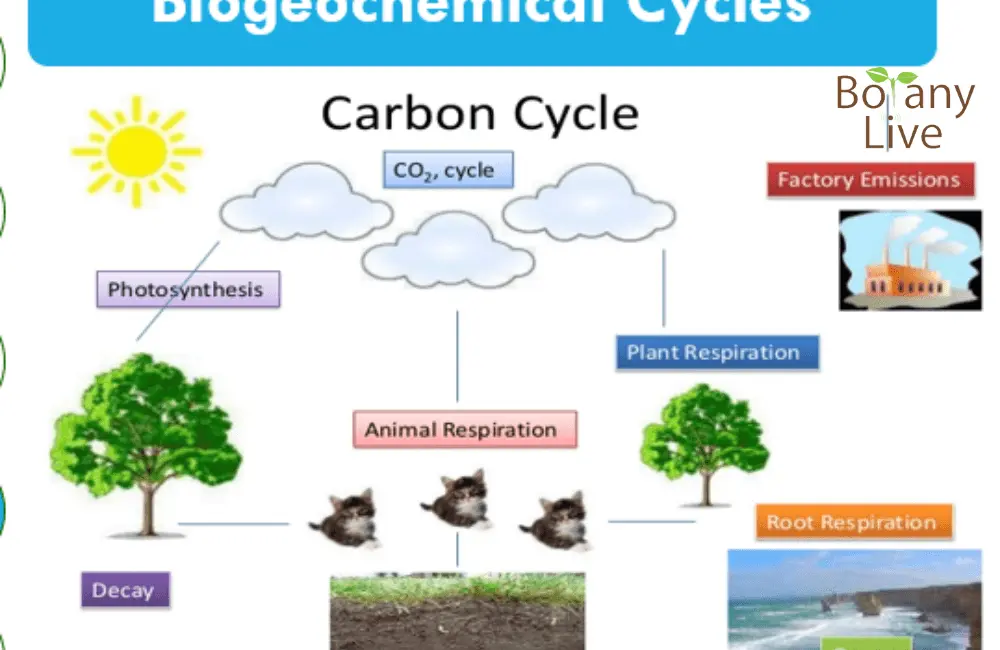
Carbon Cycle PPT
The carbon cycle is known as Carbon Chakra. The process describes how carbon circulates in biotic and abiotic components or density independent components of the ecosystem. Carbon is present in the atmosphere (0.04%), oceans, and in rocks (Limestone – CaCO3). There are following steps involved in this cyclic process.
Photosynthesis
The process of photosynthesis takes place in plants, algae, and cyanobacteria. These are the base of food chains (producers). All these organisms convert carbon into organic compounds e.g. glucose.
Respiration and Decomposition
The organic compound (glucose) is used in cellular respiration to provide energy. This compound may also pass to the next trophic level and so on. During the process of cellular respiration, Carbon is returned to the atmosphere in the form of CO2.
So, to summarize these processes, producers capture CO2, produce Glucose, use this as an input for cellular respiration, and the rest is fed to the next trophic level. The next consumer feed on this respires, or dies and the CO2 is decomposed by the decomposers and returned to the atmosphere.
Coal
If the vegetation matter is partially decomposed, it forms coal. This coal is also a storehouse of Carbon. This carbon is released into the atmosphere at the time of the complete burning and combustion process.
Marine Planktons
Some of the carbon is stored in marine plankton, unicellular organisms, and a source of soil and natural gas. This carbon is a component of fossil fuels, a source of non-renewable energy. Combustion and burning of these fossil fuels release carbon into the atmosphere.
Rocks (Limestone)
A greater amount of carbon is stored in the rocks (limestones) for millions of years. Shells of marine organisms buried deep in the ocean bed are another source of CO2. These shells may cement together to form rocks. Geological processes such as erosion and weathering slowly erode and release this carbon.
Carbon Cycle – Power Point Presentation
FAQs
I’m Dr Qaiser Maqsood (PhD), a dedicated researcher and expert in Biological Sciences, Gardening, Bio-Diversity, Ecology, and Environmental Sciences. I’m much concerned about Environmental Pollution, Climate Change, Plantation, Gardening, and Global Warming. My passion is to explore innovative solutions in all these fields.
Be aware that we have ONLY ONE EARTH. Protect it!!